Machine learning and DFT-driven development of energy materials and electrochemical devices

We are developing new methodologies based on machine learning (artificial intelligence, AI) and density functional therory (DFT) calculations to accelerate the development of energy materials and electrochemical devices. We investigate the critical descriptor for electrochemical phenomenon and plug it into a model to predict the properties of energy materials and electrochemical devices. The usage of machine learning accelerates materials development by a factor of 60 thousands in comparison with conventional ways. We, in fact, discovered a new proton-conducting materials by just one synthesis based on machine learning prediction [1, selected as the cover art and press released] and descriptor for proton conductivity [2, press released] in perovskite oxides.
Using machine learning for developing inorganic solid materials is challenging. Unlike organic materials that show a great correlation between structure and property, the existence of defects, which are the key for activating materials functionality, makes a poor correlation between structure and property in the inorganic solid materials. The successful examples of the development of inorganic solids using machine learning was limited to the optimization of chemical compositions within or between specific host compounds that had been known for a target property in the literature. Such usage, however, does not resemble the problem of discovering a wide variety of new materials.
In the current situation, we succeeded in expanding the quantitative prediction capabilities of machine learning model previously limited to the interpolated chemical compositions to the extrapolated compositions that are imperative in materials discovery and, in fact, discovered new materials. It provided significant advances over the current situations in materials informatics and materials discovery. We are getting into the new era of materials science and actively using such methodologies for the developement of new energy materials and electrochemical devices.
In our virtual laboratory tours on Youtube and our web site, you are welcomed to see our secrets to transform the methods in discovering new energy materials and developing advanced electrochemical devices to a new level.
This project is supported by the Japan Science and Technology Agency (JST) CREST (2018~2023).
References
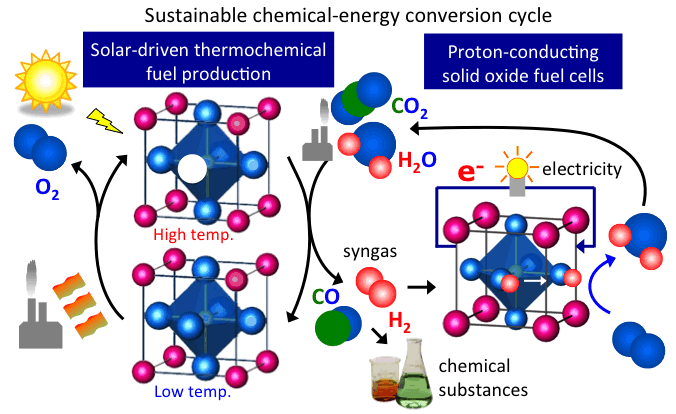
Thermochemical water splitting using perovskite oxides
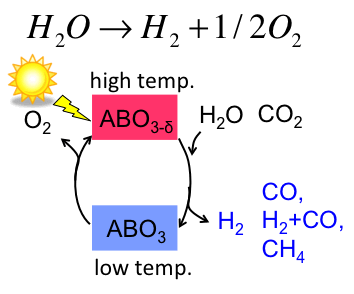
Thermochemical water splitting is a unique way to produce hydrogen from water using water dissociation. Simply, it uses two-step thermal cycle. At a higher temperature at which metal oxide is thermodynamically preferred to be reduced, oxygen would be released from the oxide while making oxygen vacant site. At a reduced temperature at which oxidation is thermodynamically preferred, the oxide will be reoxidized with water where the oxide absorbs oxygen from water molecules while releasing hydrogen to the atmosphere.
We successfully assessed such capabilities of doped perovskite and discussed a key material parameter that drives the thermochemical reaction based on thermodynamics and kinetics [1]. To further enhance the efficiency for water splitting, we focus on the characteristics of defect chemistry in the bulk and at the bulk/gas interface and their impact on such capability.
This project is supported by Japan Science and Technology Agency (JST), PRESTO (~2016) and the Japan Society for Promotion of Science (JSPS), Wakate B (2016-2018).
References
Patents
Photochemical water splitting using perovskite oxides
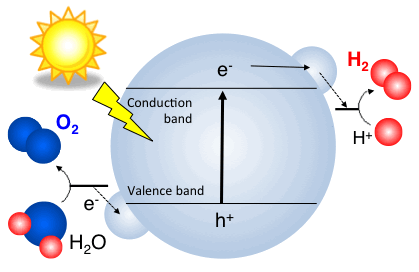
Another way to produce hydrogen from water is to use photon from sunlight. Photons are excited from the valence band to the conduction band of a photocatalyst, which would be transferred to the surface of photocatalyst and used for such reaction. The biggest challenge here is that the solar-to-fuel efficiency and its kinetics are not high enough for commercialization. To address the issues, we focus to understand what chemical and structural factor define the efficiency and kinetics [1, press released, 2-3].
References
Proton-conducting oxides for solid oxide fuel cells
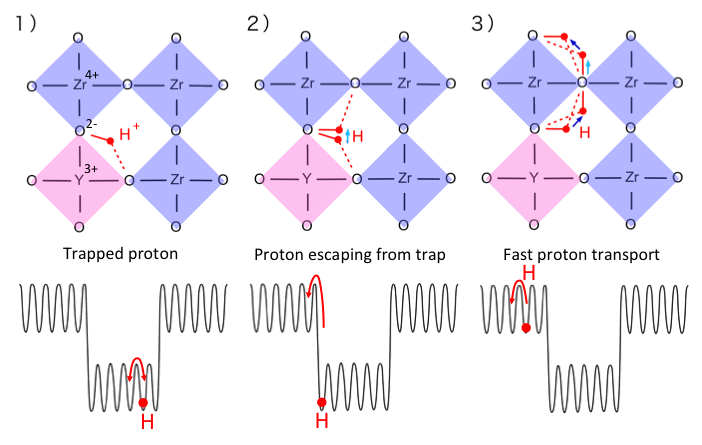
Fuel cell is a device to efficiently produce electricity from fuels. It is composed of electrolyte which permeates only ions, cathodes and anodes. We focus on proton conduction in perovskite oxides as an electrolyte. It defines the operation temperature of fuel cells.
Proton transport mechanism in the oxide is fundamental information to understand and control the proton conduction. However, the mechanism has been under debate since the discovery of proton-conduction oxide in 1981. We have demonstrated that the transport is governed by proton trapping. This fundamental understanding provided how to further enhance proton conductivity in the oxide [3, press released]. Based on the design concept proposed in our previous paper [3], we developed a fast and stable ionic materimaterials, namely the heavily Sc-doped barium zirconate (HSBZ) [1, open access and press released]. It shows high proton conductivity of 0.01 S/cm at 400 C and high chemical stability against 98% CO2 for 240 h, which is equivalent to 67 years of exposure to ambient air at the temperature. It implicates the achievement of the high performance electrochemical devices, specifically at intermediate temperatures between 300 and 450 C, which does not require any expensive materials such as platinum catalysts and high-temperature structural alloys in proton ceramic fuel cells. We are actively applying machine learning model to materials discovery and development for proton ceramic fuel cells.
This project is supported by the Japan Science and Technology Agency (JST), PRESTO (2010-2016) and the Japan Society for Promotion of Science (JSPS), Grant-in-aid for Scientific Research A (2015-2018), Shingagujutsu (2016-2018), and JST CREST (2018-2023).
References